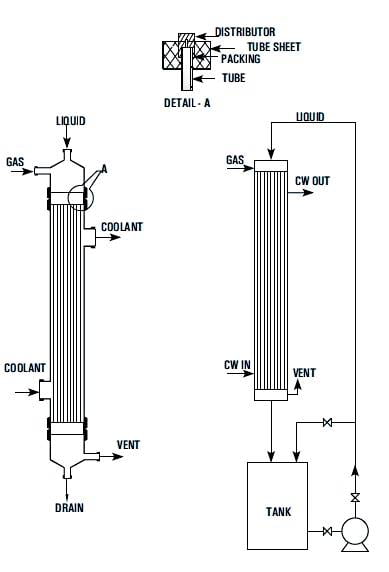
INTRODUCTION OF HCL GAS GENERATION SYSTEM

Commercial Hydrochloric Acid is available in the market as 30% aqueous solution. But for certain applications e.g. bulk drug and pharmaceuticals, HCL is required in anhydrous state for critical reactions where moisture cannot be tolerated. Such users generate anhydrous HCL fom commercial grade for their captive consumption.
METHOD OF HCL GAS GENERATOR
Several methods have been adopted by industries for HCL Gas Generator. But generation by Sulphuric Acid Route and Boiling Route are commonly practiced. We offer Calcium Chloride Route also for HCL Gas Generator.
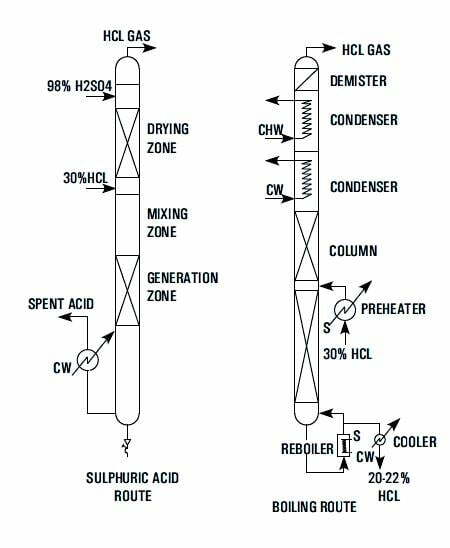
| Route | Sulphuric Acid Route | Boiling Route |
|---|---|---|
| Working Principle | Hydrochloric acid is highly soluble in water but the solubility diminishes in presence of H2SO4 and at 70 to 75% H2SO4 concentration its solubility is negligible. Thus by adding (98%) commercial Sulphuric acid to commercial hydrochloric acid (30%) in proper ratio the entire HCL can be liberated in gaseous form leaving 75% H2SO4 as spent acid. | Aqueous hydrochloric acid forms a maximum boiling point azeotrope at 110 C containing 20.24% HCL at atmospheric pressure. Thus by distilling commercial hydrochloric acid (30%) pure HCL gas can be generated and spent acid will contain over 20.24% HCL. |
| Process Outline | Metered quantities of commercial sulphuric acid hydrochloric acids are fed to the unit where they mix in the Mixing Zone. The gas generated forms a froth and enters the Generation Zone where while traveling through a bed gas is released which travels upwards through the Drying Zone. Here the gas comes in intimate contact with downward flow of 98% H2SO4. The dry gas leaving the unit passes through a rotameter. The spent liquor containing 70-75% H2SO4 passes through the Cooling Zone before being discharged. | Metered quantity of commercial hydrochloric acid is preheated in a preheater by steam and fed to a fractionating column with steam as heating media in the reboiler. The vapours leaving the column are condensed with coolant as cooling water and chilled brine in stages. The relatively dry gas passes through a mist eliminator and then through a rotameter. The spent acid containing 22% HCL is cooled through a cooler and then discharged. |
| Salient Features | – Operational reliability the unit can be started / stopped in seconds. – Available in wide range of capacities from 5 to 200 kg/hr of dry HCL. – Except cooling water no other utility e.g. steam chilled water etc. required. – Anhydrous gas. – Capable of operating from 25 to 120%. – Ease of installation. – Negligible pressure drop. – High efficiency 99%. | – Operational reliability. – Available in wide range capacities from 5 kg/hr to 200 kg/hr of dry HCL. – Except commercial hydrochloric acid, no other raw-material is required. – Anhydrous gas. – Capable of operating from 25-100%. – Ease of installation. – Negligible pressure drop. |
| Indicative Raw-material & Utilities for 20 kg/hr HCL | 30% HCL – 70 kg/hr 98% H2SO4 – 170 kg/hr Cooling Water – 2 m3 /hr | 30% HCl – 200 kg/hr Saturated Steam – 50 kg/hr Cooling Water – 3.5 m3/hr Chilled Brine – 4 m3/hr |
CALCIUM CHLORIDE ROUTE WORKING PRINCIPLE:
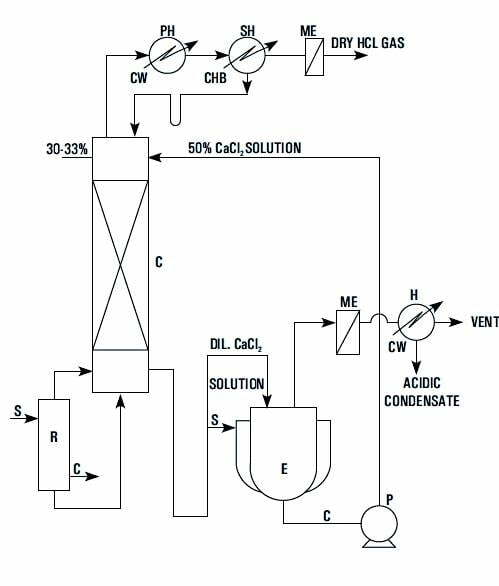
Hydrochloric acid and water form a maximum boiling point azeotrope at 11O°C corresponding to a concentration of 20.24%; (w/w) HCl. By adding concentrated CaCl solution to commercial hydrochloric acid the azeotrope 2 point is eliminated and. the entire’ HCl becomes available for liberation by distillation. Anhydrous HCl gas generation through Calcium Chloride Route is the most environmental friendly technique.
PROCESS DESCRIPTION:
The above principle- is achieved in practice by feeding metered quantities of commercial HCl and 50% CaCl -solution to a stripping column with a steam 2 heated re-boiler at bottom. The effluent from bottom of the column is a dilute acidic calcium chloride solution which is concentrated to 50% in a evaporator and re-used. The vapor leaving is condensed stage wise with cooling water and chilled brine as coolant. The relatively dry gas passes through a mist eliminator and then through a rotameter to the point of consumption.
RAW MATERIAL UTILITY REQUIREMENTS:
The indicative requirements for 20 Kg/hr HCl gas generator are given below.
| 1 | 30-32 % HCl, (Kg/hr) | : 66 |
| 2 | Cooling water at 30 °C (M /hr)) | : 4 |
| 3 | Chilled brine at -10 °C (M /hr) | : 3 |
| 4 | Steam at 6 Kg/cm (g) | : 150 |
| LEGEND | ||
| R | – | REBOILER |
| C | – | COLUMN |
| E | – | EVAPORATOR |
| PH | – | PRIMARY CONDENSER |
| SH | – | SECONDARY CONDENSER |
| H | – | CONDENSER |
| ME | – | MIST ELIMENATOR |
| P | – | PUMP |
| CW | – | COOLING WATER |
| CHB | – | CHILLED DRAINE |
| S | – | STEAM |
| C | – | CONDENSATE |